Written by: Julia Westbrook, L.E “The Beautifier”

SkinPen: The world’s first FDA approved Microneedling device!
The first recorded microneedling procedure was in 1905 performed by German Dermatologist, Ernst Kromayer, using various-sized dental burs powered by motor driven equipment to treat scars, birthmarks and hyperpigmentation. Microneedling as we now know it came to play by 1995 when Dr. Desmond Fernandes in Philadelphia used hypodermic needles, and developed a small needle stamp to induce collagen production. Now, in 2020, most people have received a treatment, know someone who has, or have at least heard of microneedling. A few years ago, Kim Kardashian made it popular and the treatment everyone wanted by showing pictures of herself receiving the famous “vampire facial”!
Let’s learn about Microneedling…
Microneedling is a skin repair and rejuvenation treatment that should be performed only by licensed medical professionals and approved (by a medical director) licensed Aesthetician’s. A mechanical device with multiple tiny needles is used to create controlled micro-injuries that stimulate the body’s natural wound healing process. Since this treatment stimulates the body’s collagen production, microneedling has also been referred to as “collagen induction therapy”. Microneedling addresses many skin complaints, the most popular being: acne scarring, wrinkles, loose skin, stretch marks and skin discoloration.
What makes SkinPen unique and better than the rest…
The SkinPen from Bellus Medical set a new technology and safety standard for microneedling, as it is the first and ONLY microneedling system to receive clearance as a Class II medical device by the FDA. The SkinPen’s state-of-the-art skin rejuvenating technology offers a minimally invasive, non-ablative option with little downtime. On the technology side, SkinPen is notable for its Advanced Microneedle Cartridge, this single-use cartridge includes a safety feature that ensures fluids, serums and blood generated during the procedure do not clog the cartridge or enter the body of the device, thus reducing risk of cross contamination. The FDA also cleared SkinPens very own hydrogel, Skinfuse Lift HG, which is used to lubricate the skin for a smooth easy glide across the face. This hydrogel is free of harmful ingredients, preventing foreign body reactions. The best part of it all, SkinPen is safe for all skin types, is clinically shown to safely and effectively treat facial acne scars for ages 22 and up, and 90% of subjects in the clinical trial would recommend the treatment to family and friends!
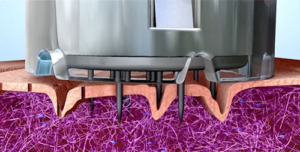
What to expect…
Immediately post-procedure, the skin will appear slightly pink to red, similar to a mild to moderate sunburn. The most common post treatment reactions experienced were dryness, discomfort, itching, peeling, redness, tightness, dry skin and burning. Don’t let that scare you away, these conditions subside and are resolved over minimal time and the results are WORTH IT! Over the next couple of weeks post microneedling treatment, you can expect the skin to feel and appear tighter, lifted, brighter and smoother.

Before and Immediately post procedure
*images property of L-Aesthetics and Longevity
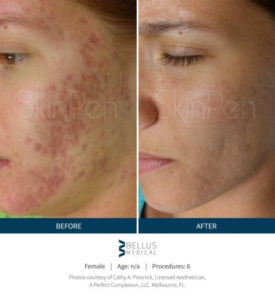
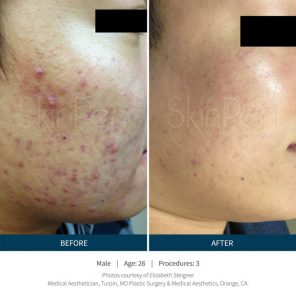
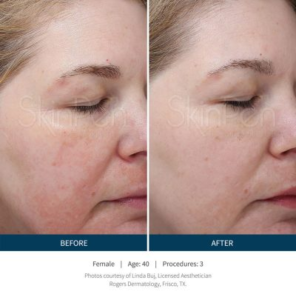
SkinPen results…
“See for yourself why microneedling is a growing part of the 2.5 million annual skin rejuvenation and renewal procedures worldwide in our gallery of SkinPen pre- and post-procedure® photos.” 1-References
[1] Singh A., Yadav S. Microneedling: Advances and Widening Horizons. Indian Dermatology Online Journal. 2016 Skinpen.com